CALL US
TO BOOK AN
APPOINTMENT
(770) 771-6591



Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan
Indications, Important Safety Information and Prescribing
Information
BOTOX® Cosmetic
(onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is
indicated in adult patients for the temporary improvement in the appearance of:
- Moderate to severe glabellar lines associated with
corrugator and/or procerus muscle activity
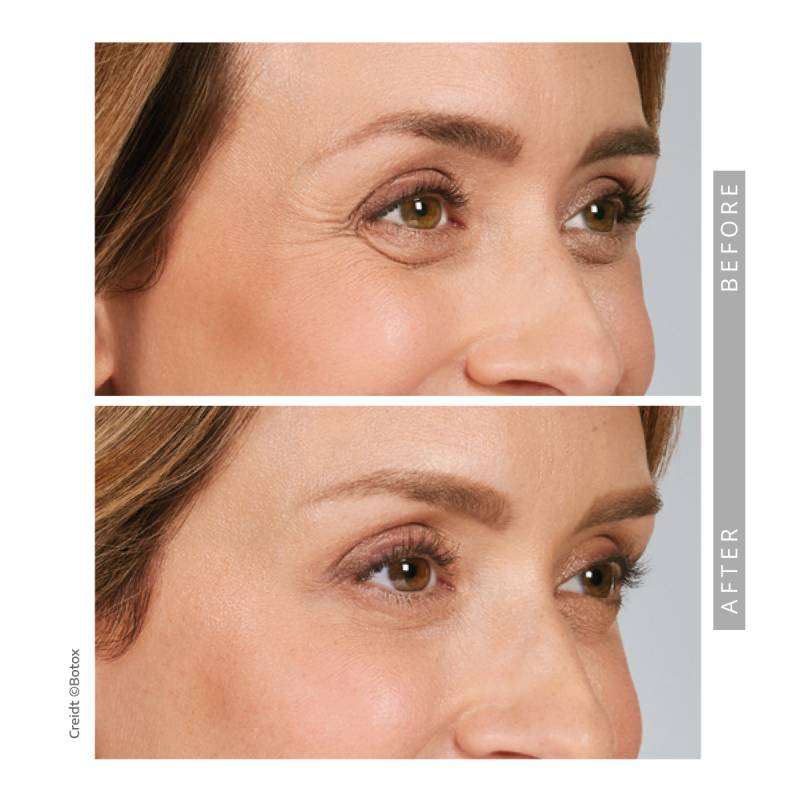
- Moderate to severe lateral canthal lines associated with
orbicularis oculi activity
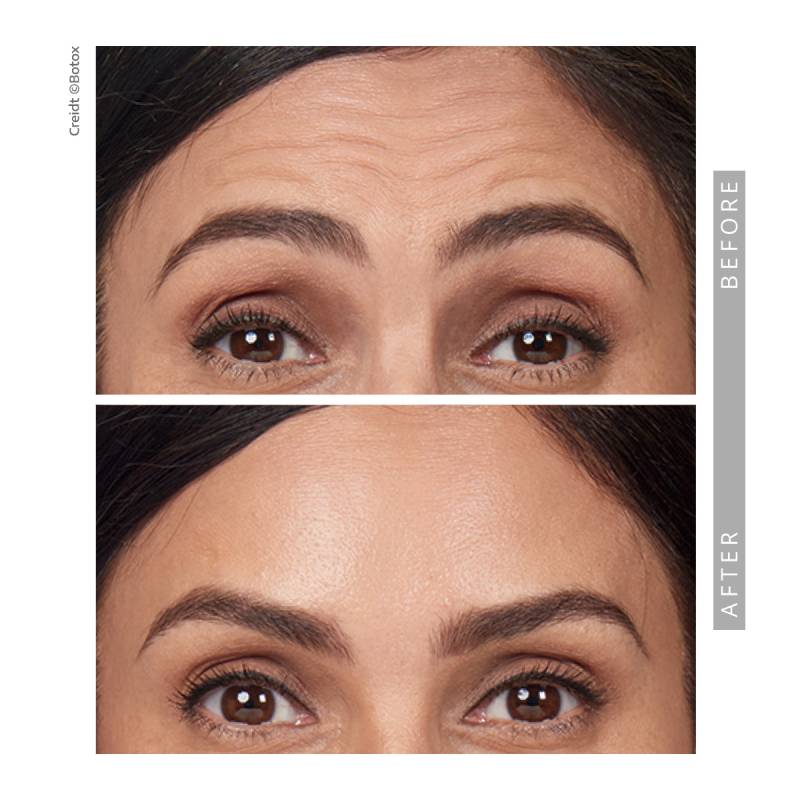
- Moderate to severe forehead lines associated with frontalis
activity
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic
and all botulinum toxin products may spread from the area of injection to
produce symptoms consistent with botulinum toxin effects. These may include
asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia,
dysarthria, urinary incontinence, and breathing difficulties. These symptoms
have been reported hours to weeks after injection. Swallowing and breathing
difficulties can be life threatening and there have been reports of death. The
risk of symptoms is probably greatest in children treated for spasticity, but
symptoms can also occur in adults treated for spasticity and other conditions,
particularly in those patients who have an underlying condition that would
predispose them to these symptoms. In unapproved uses and approved indications,
cases of spread of effect have been reported at doses comparable to those used
to treat cervical dystonia and spasticity and at lower doses.
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the
presence of infection at the proposed injection site(s) and in individuals with
known hypersensitivity to any botulinum toxin preparation or to any of the
components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin
Products
The potency units of BOTOX® Cosmetic are
specific to the preparation and assay method utilized. They are not interchangeable
with other preparations of botulinum toxin products and, therefore, units of
biological activity of BOTOX® Cosmetic cannot be compared to
nor converted into units of any other botulinum toxin products assessed with
any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin
Effect.
No definitive serious adverse event reports of distant
spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic
at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral
canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units
(for simultaneous treatment of lateral canthal lines and glabellar lines), and
64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines,
and forehead lines) have been reported. Patients or caregivers should be
advised to seek immediate medical care if swallowing, speech, or respiratory
disorders occur.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness,
dysphagia, and aspiration pneumonia, with some adverse reactions associated
with fatal outcomes, have been reported in patients who received BOTOX® injections
for unapproved uses. In these cases, the adverse reactions were not necessarily
related to distant spread of toxin, but may have resulted from the
administration of BOTOX® to the site of injection and/or
adjacent structures. In several of the cases, patients had pre-existing
dysphagia or other significant disabilities. There is insufficient information
to identify factors associated with an increased risk for adverse reactions
associated with the unapproved uses of BOTOX®. The safety and
effectiveness of BOTOX® for unapproved uses have not been
established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have
been reported. These reactions include anaphylaxis, serum sickness, urticaria,
soft-tissue edema, and dyspnea. If such reactions occur, further injection of
BOTOX® Cosmetic should be discontinued and appropriate medical
therapy immediately instituted. One fatal case of anaphylaxis has been reported
in which lidocaine was used as the diluent and, consequently, the causal agent
cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of
adverse events involving the cardiovascular system, including arrhythmia and
myocardial infarction, some with fatal outcomes. Some of these patients had
risk factors including pre-existing cardiovascular disease. Use caution when
administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects With
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases,
amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg,
myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given
botulinum toxin. Patients with neuromuscular disorders may be at increased risk
of clinically significant effects including generalized muscle weakness,
diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory
compromise from onabotulinumtoxinA (see Warnings and
Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® and other botulinum
toxin products can result in swallowing or breathing difficulties. Patients
with pre-existing swallowing or breathing difficulties may be more susceptible
to these complications. In most cases, this is a consequence of weakening of
muscles in the area of injection that are involved in breathing or
oropharyngeal muscles that control swallowing or breathing (see Boxed
Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic
treatment is used in the presence of inflammation at the proposed injection
site(s) or when excessive weakness or atrophy is present in the target
muscle(s).
Dry Eye in Patients Treated With BOTOX® Cosmetic
There have been reports of dry eye associated with BOTOX® Cosmetic
injection in or near the orbicularis oculi muscle. If symptoms of dry eye (eg,
eye irritation, photophobia, or visual changes) persist, consider referring
patients to an ophthalmologist.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood.
Based on effective donor screening and product manufacturing processes, it
carries an extremely remote risk for transmission of viral diseases and variant
Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission
of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk
of transmission would also be considered extremely remote. No cases of
transmission of viral diseases, CJD or vCJD have ever been identified for
licensed albumin or albumin contained in other licensed products.
ADVERSE REACTIONS
The most frequently reported adverse reactions following
injection of BOTOX® Cosmetic for glabellar lines were eyelid
ptosis (3%), facial pain (1%), facial paresis (1%), and muscular weakness (1%).
The most frequently reported adverse reaction following
injection of BOTOX® Cosmetic for lateral canthal lines was
eyelid edema (1%).
The most frequently reported adverse reactions following
injection of BOTOX® Cosmetic for forehead lines with glabellar
lines were headache (9%), brow ptosis (2%), and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and
aminoglycosides or other agents interfering with neuromuscular transmission
(eg, curare-like compounds) should only be performed with caution as the effect
of the toxin may be potentiated. Use of anticholinergic drugs after
administration of BOTOX® Cosmetic may potentiate systemic
anticholinergic effects.
The effect of administering different botulinum neurotoxin
products at the same time or within several months of each other is unknown.
Excessive neuromuscular weakness may be exacerbated by
administration of another botulinum toxin prior to the
resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration
of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing
surveillance on the developmental risk associated with use of BOTOX® Cosmetic
in pregnant women. There are no data on the presence of BOTOX® Cosmetic
in human or animal milk, the effects on the breastfed child, or the effects on
milk production.
Please see BOTOX® Cosmetic
full Prescribing
Information including Boxed Warning and Medication Guide.
Neurotoxins, redefined.
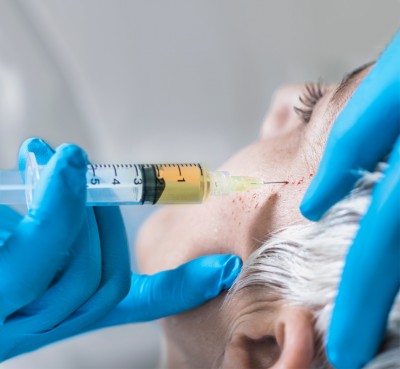
We’re here to reduce frown lines, crow’s feet, wrinkles, other signs of aging, pores, redness, and more. Looking for Botox, Dysport, Xeomin, or other neurotoxin injections in Johns Creek North Atlanta? Then visit us at Johns Creek Dermatology so we can advise you on the best cosmetic non-invasive non-surgical solution for your needs.
• Fresh Botox, Dysport, Xeomin is diluted for the highest efficacy per FDA standard.
WE OFFER
Botox, Dysport, Xeomin, and the list goes on as newer neuromodulators are developed, most notably longer-acting ones that last at least two times longer than older neurotoxins (i.e. Daxi).
We have
long excelled at the classic applications of neurotoxins for frown lines,
forehead lines and crow's feet, but our expertise and skilled physicians who
are master injectors mean we can also offer advanced techniques that are
constantly evolving.
We accept the Allē Loyalty Program for Botox, Juvéderm, and Skinvive by Juvéderm.
Facial
Contouring “Slim Your Face”
Did you know botulinum toxin can be used to recontour and slim the face? This treatment is especially effective for relaxing the larger muscles of the lower cheeks to provide a more feminine, V-shaped face.
Lip Lift “Turn Your Frown Upside Down”
We all know that as we age our faces lose fullness and begin to droop. Unfortunately, the same changes occur in our lips. Surgical options can be costly and invasive. A non-surgical lip lift with neurotoxins can reverse the downward frown appearance and can evert the lips for a pouty more attractive look.
Nefertiti Neck Lift
The Nefertiti Neck Lift is named after Queen Nefertiti whose bone structure has been admired for centuries. The Nefertiti Neck Lift is a cutting-edge technique that utilizes a series of Botox injections along the neck and jawline. This is a safe and non-surgical technique. The net effect is an upward pull from facial muscles resulting in a more smooth and defined jawline.
Pole Size Reduction
This is an effective and safe treatment to shrink pore size. Micro-doses of neurotoxin (aka “Micro-tox”) can be injected directly into the pores by a master injector technique or via the Aquagold Facial device. The neurotoxin acts on the arrector pili muscles to constrict and shrink pore size, while also reducing oil production.
Flushing and Redness Reduction
“Micro-tox” can also be used to reduce redness and flushing in the skin, which is extremely beneficial for our many patients with rosacea. Administered via an injection or the Aquagold facial device, micro-tox blocks inflammatory markers in the skin and inhibits the autonomic peripheral nerves of the cutaneous vascular system, leading to significant reduction in redness and control of facial flushing.
FREQUENTLY ASKED QUESTIONS
What is BOTOX cosmetic?
This is an FDA-approved injectable to treat wrinkles between the eyebrows, in the forehead and in the eye area.
How much research has gone into BOTOX cosmetic?
This is a treatment that has been used for many years. To date millions of people have received this injectable.
How does BOTOX cosmetic work?
It is a neurotoxin that blocks the ability of a muscle to contract when ordered by a nerve. Thus, it is most effective with wrinkles that are induced by gestures and less effective for wrinkles that are visible at rest.
Is there a substitute for BOTOX cosmetic?
There are no generics, however there are other brand names such as Dysport, Xeomin, and Juveua. They have subtle differences in the efficacy and results. Your dermatologist will advise you on the ideal product.
Will my face look overdone or unnatural?
You can look very natural and youthful, make sure you explain your goals very well and make sure you consult with a dermatologist that is willing to listen. I advise against overdone looks.
How can I save on Botox cosmetic?
Be wary of unbelievable deals because they are probably too good to be true. Be sure you are being injected by a board-certified dermatologist who follows the standard of care for handling and reconstituting the product
Is Botox cosmetic only for women?
Absolutely not! Men are more conscious than ever of the aging process especially in this competitive world. We are more visible than ever and are connecting from the comfort of our home on zoom and making many more business and social networks.
Do I need to be over 40 to start using Botox cosmetic?
Many patients younger than 30 are getting these treatments for prevention, especially if they have family history of pesky wrinkles.
How much time does Botox cosmetic treatment take?
The treatment itself is quick, but the consult for first time injectors takes time. Even for repeat patients we have to reset the goals depending on the patient’s needs. The plan always evolves and never stays static. Aging is not static.
Will it hurt?
The injections are usually very well tolerated. But there are strategies to make them extremely acceptable. Don’t be shy to discuss your concerns about needles and discomfort with your dermatologist.
Will I see results quickly? How long does BOTOX cosmetic last?
I advise my patients that they will start seeing noticeable changes around 48 hours. Usually the result is optimal around 2 weeks. The injections last approximately 3-4 months depending on the patient’s metabolism.
How long is the recovery time after treatment?
There is minimal to no downtime, there are some precautions that are important to enhance safety of the treatments and maximize its benefit.
How many injections will I receive?
Patients are very unique and treatment plans vary. There are no set injections or units. Herein lies the difference between a seasoned dermatologist and other injectors.
What were common side effects seen in clinical studies?
All medical and cosmetic treatments have risks and benefits. Not every patient is a candidate. My approach is never to allow the risks to overweigh the benefits. These risks are accessible to you online but it is ideal to discuss them with your dermatologist, their experience is invaluable.





Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan
Indications, Important Safety Information and Prescribing
Information
BOTOX® Cosmetic
(onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is
indicated in adult patients for the temporary improvement in the appearance of:
- Moderate to severe glabellar lines associated with
corrugator and/or procerus muscle activity
- Moderate to severe lateral canthal lines associated with
orbicularis oculi activity
- Moderate to severe forehead lines associated with frontalis
activity
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic
and all botulinum toxin products may spread from the area of injection to
produce symptoms consistent with botulinum toxin effects. These may include
asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia,
dysarthria, urinary incontinence, and breathing difficulties. These symptoms
have been reported hours to weeks after injection. Swallowing and breathing
difficulties can be life threatening and there have been reports of death. The
risk of symptoms is probably greatest in children treated for spasticity, but
symptoms can also occur in adults treated for spasticity and other conditions,
particularly in those patients who have an underlying condition that would
predispose them to these symptoms. In unapproved uses and approved indications,
cases of spread of effect have been reported at doses comparable to those used
to treat cervical dystonia and spasticity and at lower doses.
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the
presence of infection at the proposed injection site(s) and in individuals with
known hypersensitivity to any botulinum toxin preparation or to any of the
components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin
Products
The potency units of BOTOX® Cosmetic are
specific to the preparation and assay method utilized. They are not interchangeable
with other preparations of botulinum toxin products and, therefore, units of
biological activity of BOTOX® Cosmetic cannot be compared to
nor converted into units of any other botulinum toxin products assessed with
any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin
Effect.
No definitive serious adverse event reports of distant
spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic
at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral
canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units
(for simultaneous treatment of lateral canthal lines and glabellar lines), and
64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines,
and forehead lines) have been reported. Patients or caregivers should be
advised to seek immediate medical care if swallowing, speech, or respiratory
disorders occur.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness,
dysphagia, and aspiration pneumonia, with some adverse reactions associated
with fatal outcomes, have been reported in patients who received BOTOX® injections
for unapproved uses. In these cases, the adverse reactions were not necessarily
related to distant spread of toxin, but may have resulted from the
administration of BOTOX® to the site of injection and/or
adjacent structures. In several of the cases, patients had pre-existing
dysphagia or other significant disabilities. There is insufficient information
to identify factors associated with an increased risk for adverse reactions
associated with the unapproved uses of BOTOX®. The safety and
effectiveness of BOTOX® for unapproved uses have not been
established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have
been reported. These reactions include anaphylaxis, serum sickness, urticaria,
soft-tissue edema, and dyspnea. If such reactions occur, further injection of
BOTOX® Cosmetic should be discontinued and appropriate medical
therapy immediately instituted. One fatal case of anaphylaxis has been reported
in which lidocaine was used as the diluent and, consequently, the causal agent
cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of
adverse events involving the cardiovascular system, including arrhythmia and
myocardial infarction, some with fatal outcomes. Some of these patients had
risk factors including pre-existing cardiovascular disease. Use caution when
administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects With
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases,
amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg,
myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given
botulinum toxin. Patients with neuromuscular disorders may be at increased risk
of clinically significant effects including generalized muscle weakness,
diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory
compromise from onabotulinumtoxinA (see Warnings and
Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® and other botulinum
toxin products can result in swallowing or breathing difficulties. Patients
with pre-existing swallowing or breathing difficulties may be more susceptible
to these complications. In most cases, this is a consequence of weakening of
muscles in the area of injection that are involved in breathing or
oropharyngeal muscles that control swallowing or breathing (see Boxed
Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic
treatment is used in the presence of inflammation at the proposed injection
site(s) or when excessive weakness or atrophy is present in the target
muscle(s).
Dry Eye in Patients Treated With BOTOX® Cosmetic
There have been reports of dry eye associated with BOTOX® Cosmetic
injection in or near the orbicularis oculi muscle. If symptoms of dry eye (eg,
eye irritation, photophobia, or visual changes) persist, consider referring
patients to an ophthalmologist.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood.
Based on effective donor screening and product manufacturing processes, it
carries an extremely remote risk for transmission of viral diseases and variant
Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission
of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk
of transmission would also be considered extremely remote. No cases of
transmission of viral diseases, CJD or vCJD have ever been identified for
licensed albumin or albumin contained in other licensed products.
ADVERSE REACTIONS
The most frequently reported adverse reactions following
injection of BOTOX® Cosmetic for glabellar lines were eyelid
ptosis (3%), facial pain (1%), facial paresis (1%), and muscular weakness (1%).
The most frequently reported adverse reaction following
injection of BOTOX® Cosmetic for lateral canthal lines was
eyelid edema (1%).
The most frequently reported adverse reactions following
injection of BOTOX® Cosmetic for forehead lines with glabellar
lines were headache (9%), brow ptosis (2%), and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and
aminoglycosides or other agents interfering with neuromuscular transmission
(eg, curare-like compounds) should only be performed with caution as the effect
of the toxin may be potentiated. Use of anticholinergic drugs after
administration of BOTOX® Cosmetic may potentiate systemic
anticholinergic effects.
The effect of administering different botulinum neurotoxin
products at the same time or within several months of each other is unknown.
Excessive neuromuscular weakness may be exacerbated by
administration of another botulinum toxin prior to the
resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration
of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing
surveillance on the developmental risk associated with use of BOTOX® Cosmetic
in pregnant women. There are no data on the presence of BOTOX® Cosmetic
in human or animal milk, the effects on the breastfed child, or the effects on
milk production.
Please see BOTOX® Cosmetic
full Prescribing
Information including Boxed Warning and Medication Guide.