Nikki Carmen - Facebook Review
Johns Creek Derm... did my filler and I’m absolutely in love! Highly recommend them for any cosmetic needs!!
PATIENT REVIEWS


Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan
JUVÉDERM® Collection of
Fillers Important Information
INDICATIONS
JUVÉDERM® VOLUMA™ XC injectable gel is indicated for deep (subcutaneous
and/or supraperiosteal) injection for cheek augmentation to correct age-related
volume deficit in the mid-face in adults over the age of 21.
JUVÉDERM® VOLLURE™ XC injectable gel is indicated
for injection into the mid-to-deep dermis for correction of moderate to severe
facial wrinkles and folds (such as nasolabial folds) in adults over the age of
21.
JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC injectable gels are indicated
for injection into the mid-to-deep dermis for correction of moderate to severe
facial wrinkles and folds (such as nasolabial folds).
JUVÉDERM® VOLBELLA™ XC injectable gel is indicated for
injection into the lips for lip augmentation and for correction of perioral
rhytids in adults over the age of 21.
JUVÉDERM® Ultra XC injectable gel is indicated for
injection into the lips and perioral area for lip augmentation in adults over
the age of 21.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to Gram-positive bacterial proteins or lidocaine contained in these products.
WARNINGS
- Do not inject
into blood vessels. Introduction of these products into the vasculature
may lead to embolization, occlusion of the vessels, ischemia, or
infarction. Take extra care when injecting soft-tissue fillers; for
example, inject the product slowly and apply the least amount of pressure
necessary. Rare, but serious, adverse events associated with the
intravascular injection of soft-tissue fillers in the face have been
reported and include temporary or permanent vision impairment, blindness,
cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis,
and damage to underlying facial structures. Immediately stop the injection
if a patient exhibits any of the following symptoms: changes in vision,
signs of a stroke, blanching of the skin, unusual pain during or shortly
after the procedure. Patients should receive prompt medical attention and,
possibly, evaluation by an appropriate healthcare professional specialist
should an intravascular injection occur
- Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
- To minimize the
risk of potential complications, these products should only be used by
healthcare professionals who have appropriate training, experience, and
knowledge of facial anatomy
- Healthcare
professionals are encouraged to discuss the potential risks of soft-tissue
injections with their patients prior to treatment and ensure that patients
are aware of signs and symptoms of potential complications
- The safety and
effectiveness for the treatment of anatomic regions other than the
mid-face with JUVÉDERM® VOLUMA™ XC; facial
wrinkles and folds with JUVÉDERM® Ultra XC,
JUVÉDERM® Ultra Plus
XC, and JUVÉDERM® VOLLURE™
XC; and the lips and perioral area with JUVÉDERM® Ultra XC
and JUVÉDERM® VOLBELLA™ XC have
not been established in controlled clinical studies
- As with all
transcutaneous procedures, dermal filler implantation carries a risk of
infection. Follow standard precautions associated with injectable
materials
- The safety for
use during pregnancy, in breastfeeding females, and in patients with known
susceptibility to keloid formation, hypertrophic scarring, and pigmentation
disorders has not been studied
- The safety for
use of JUVÉDERM® VOLUMA™ XC in
patients under 35 or over 65 years, JUVÉDERM® Ultra XC
and JUVÉDERM® Ultra Plus
XC in patients under 18 years, and JUVÉDERM® VOLLURE™
XC and JUVÉDERM® VOLBELLA™ XC in patients
under 22 years has not been established
- Use with caution
in patients on immunosuppressive therapy
- Patients who are
using products that can prolong bleeding (such as aspirin, nonsteroidal
anti-inflammatory drugs, and warfarin) may experience increased bruising
or bleeding at treatment sites
- If laser
treatment, chemical peel, or any other procedure based on active dermal
response is considered after treatment, or if these products are
administered before the skin has healed completely, there is a possible
risk of an inflammatory reaction at the treatment site
- Patients who
experience skin injury near the site of implantation may be at a higher
risk for adverse events
- The safety of
JUVÉDERM® VOLUMA™ XC
injectable gel for use in patients with very thin skin in the mid-face and
Fitzpatrick Skin Types V and VI has not been established
- Patients may
experience late onset nodules with use of dermal fillers including
JUVÉDERM® VOLUMA™ XC
- Patients may
experience late onset adverse events with use of dermal fillers
ADVERSE EVENTS
The most commonly
reported side effects for JUVÉDERM® injectable
gels were injection-site redness, swelling, pain, tenderness, firmness,
lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA™ XC,
dryness was also reported. For JUVÉDERM® VOLUMA™ XC,
side effects were predominantly moderate in severity, with duration of 2 to 4
weeks; for JUVÉDERM® Ultra XC , JUVÉDERM® Ultra
Plus XC, or JUVÉDERM® VOLLURE™ XC, they
were mostly mild or moderate in severity, with duration of 14 days or less; and
for JUVÉDERM® VOLBELLA™ XC,
they were predominantly mild or moderate, with duration of 30 days or less.
To report an adverse reaction with any product in the JUVÉDERM® Collection, please call Allergan at 1-800-433-8871. Please visit JuvedermDFU.com for more information.
Products in the JUVÉDERM® Collection are available by prescription only.
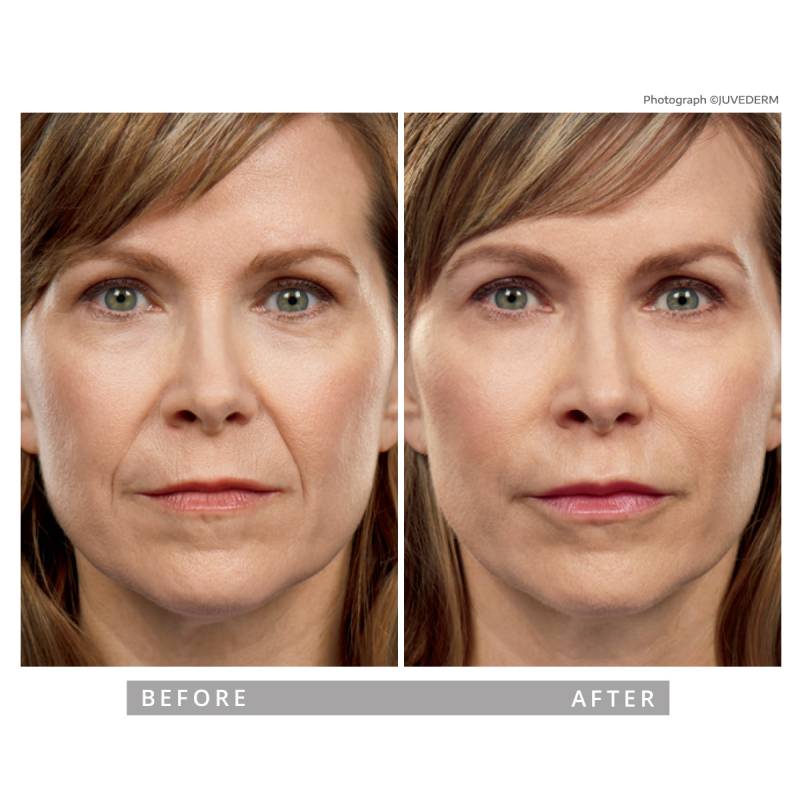
Lift, Plump, Sculpt, and Refresh—Without Looking Overdone
Why Choose Us for Dermal Fillers?
Expert Injections by a Triple Board-Certified Dermatologist
Tailored to Your Facial Structure
Realistic, Beautiful Outcomes
Commitment to Safety
Transparent Pricing
Trusted Dermal Filler Brands
Juvéderm®
Restylane®
Sculptra®
Radiesse®
Customized Lip, Cheek, Under-Eye & Facial Filler Treatments
Classic Rejuvenation
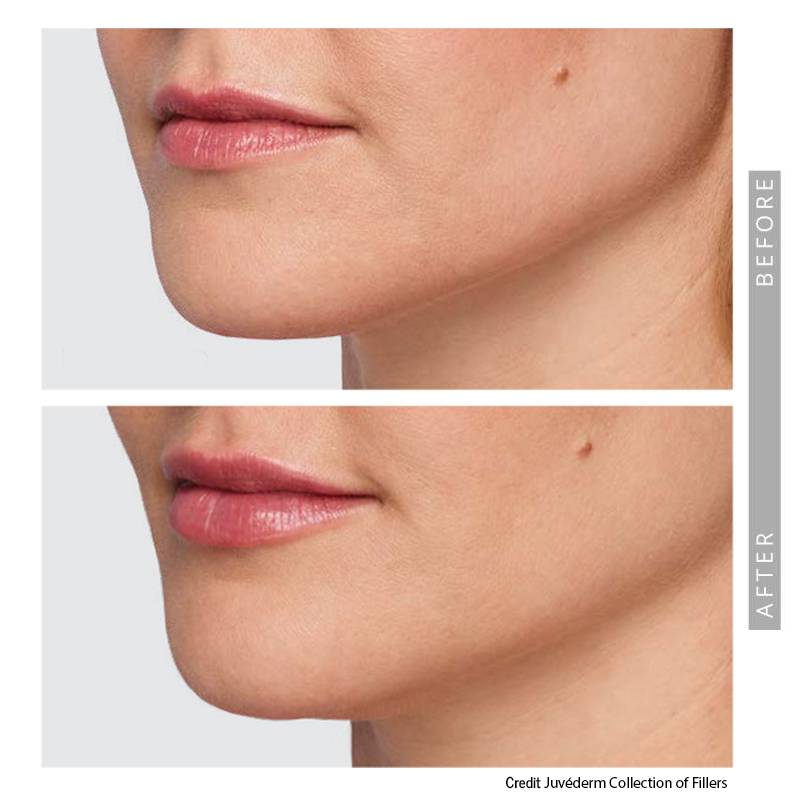
Lip Fillers
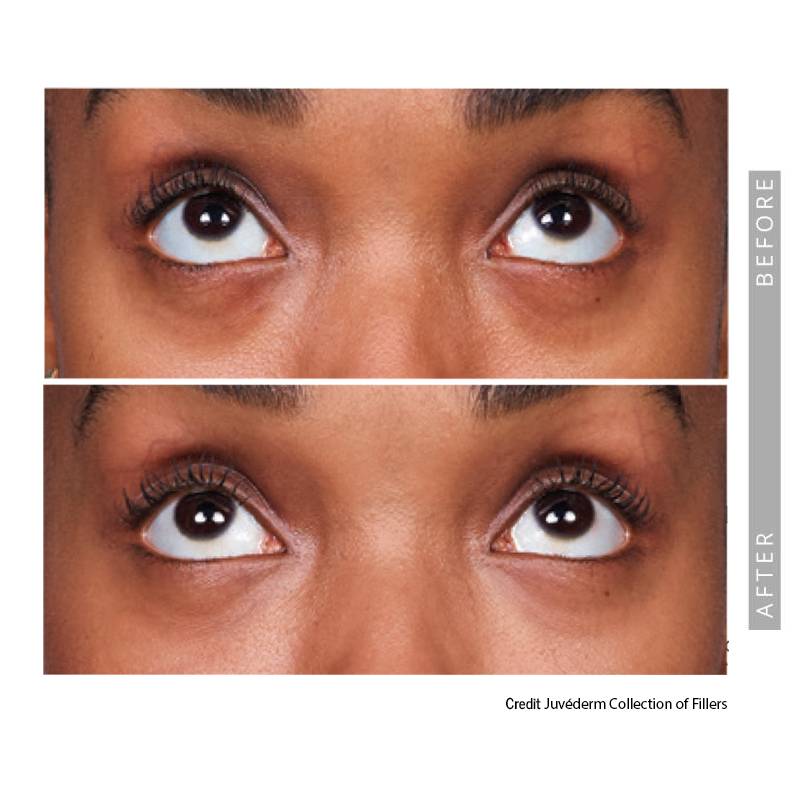
Under-Eye Fillers
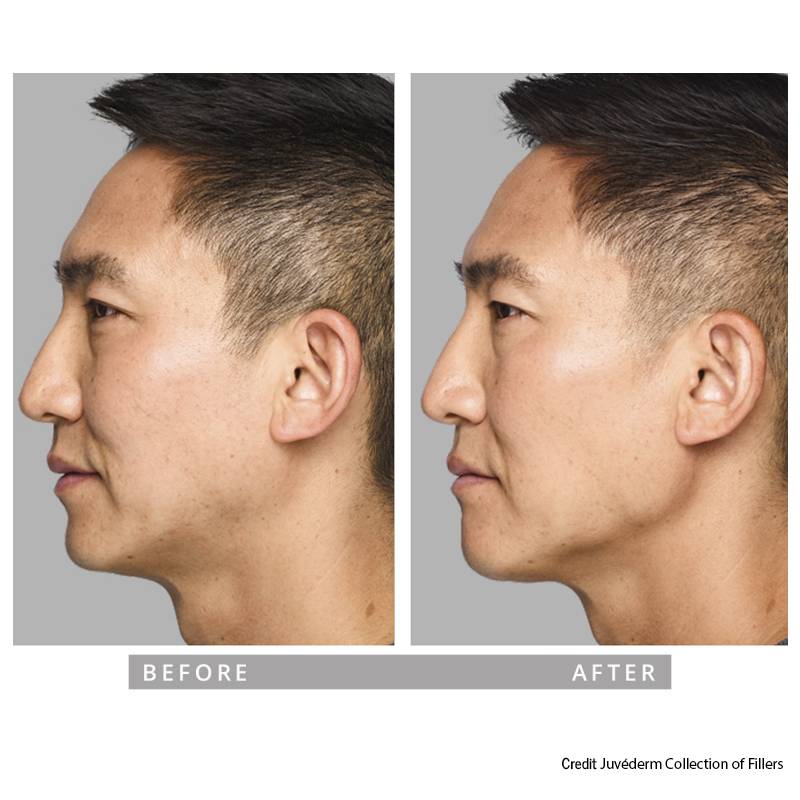
Cheek & Mid-face Contouring
The "Top Model Look"
Non-Surgical Lifts
Glowing Skin
Book Your Personalized Dermal Filler Consultation
Dermal Filler Frequently Asked Questions
What are fillers?
These are different formulations in injections that are FDA approved to lift wrinkles, volumize cheeks, re- contour the face, or plump the lips.
What do they look like?
They range from transparent gels to milky foamy solutions, depending on the type of the fillers. Different fillers have different characteristics and can achieve different goals. They are all amazing if used properly. Hyaluronic acid-based products tend to be common. Please take time look at the syringe before it’s injected, it’s fun and satisfying.
What is Hyaluronic Acid, aka HA?
HA is a natural substance in the skin that delivers nutrients, helps the skin retain moisture and softness, and adds volume. The fillers in this special are composed of a very unique formulation of this material, providing a natural look, with less swelling and to top it off they have amazing lifespan and longevity.
I like products based on HA’s because they are reversible if needed.
What is the treatment experience like?
I understand the apprehension of new comers, that’s why proper education at the first consult is critical. Most patients’ fears minimize after the consult and disappear after the very first injection. The cutting-edge techniques allow Board certified Dermatologists to inject the product more safely with the least amount for discomfort.
Will I look like myself?
That should be the goal of the treatment, a better more youthful version of yourself not a different or distorted version. I strongly believe that a patient should be allowed to look at the results multiple times between the injections. Conservative approaches are the safest approaches. Add-ons are easy, reversals are more challenging.
When will i see results?
Results are immediate but occasionally can be blurred by swelling. I prefer fillers in these that retain less selling so usually what you see is what you get.
What is the average treatment cost?
Look for someone who is licensed and trained and has experience treating patients with the fillers and Be wary of discount products or “cheap” treatments —if it sounds too good to be true, it probably is. Sterility is critical, intact syringes is a sacred rule.
How long do results last?
This can vary depending on the patient’s history, metabolism and the product itself. For new comers I recommend starting with dermal fillers that last around 6 months and then graduating to fillers that last longer so they can get a feel for the treatments
What is the reason I should have filler?
Yes, not all patients are candidates, all fillers have potential side effects and some can be serious. These are very important to disclose prior to the injection. Millions of patients have received dermal fillers over the course of years, Fortunately the concerning side effects remain unusual but a statistic is a statistic it can happen. I am very religious with making sure that patients understand these very well.
READ MORE ABOUT
Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan
JUVÉDERM® Collection of
Fillers Important Information
INDICATIONS
JUVÉDERM® VOLUMA™ XC injectable gel is indicated for deep (subcutaneous
and/or supraperiosteal) injection for cheek augmentation to correct age-related
volume deficit in the mid-face in adults over the age of 21.
JUVÉDERM® VOLLURE™ XC injectable gel is indicated
for injection into the mid-to-deep dermis for correction of moderate to severe
facial wrinkles and folds (such as nasolabial folds) in adults over the age of
21.
JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC injectable gels are indicated
for injection into the mid-to-deep dermis for correction of moderate to severe
facial wrinkles and folds (such as nasolabial folds).
JUVÉDERM® VOLBELLA™ XC injectable gel is indicated for
injection into the lips for lip augmentation and for correction of perioral
rhytids in adults over the age of 21.
JUVÉDERM® Ultra XC injectable gel is indicated for
injection into the lips and perioral area for lip augmentation in adults over
the age of 21.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to Gram-positive bacterial proteins or lidocaine contained in these products.
WARNINGS
- Do not inject
into blood vessels. Introduction of these products into the vasculature
may lead to embolization, occlusion of the vessels, ischemia, or
infarction. Take extra care when injecting soft-tissue fillers; for
example, inject the product slowly and apply the least amount of pressure
necessary. Rare, but serious, adverse events associated with the
intravascular injection of soft-tissue fillers in the face have been
reported and include temporary or permanent vision impairment, blindness,
cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis,
and damage to underlying facial structures. Immediately stop the injection
if a patient exhibits any of the following symptoms: changes in vision,
signs of a stroke, blanching of the skin, unusual pain during or shortly
after the procedure. Patients should receive prompt medical attention and,
possibly, evaluation by an appropriate healthcare professional specialist
should an intravascular injection occur
- Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
- To minimize the
risk of potential complications, these products should only be used by
healthcare professionals who have appropriate training, experience, and
knowledge of facial anatomy
- Healthcare
professionals are encouraged to discuss the potential risks of soft-tissue
injections with their patients prior to treatment and ensure that patients
are aware of signs and symptoms of potential complications
- The safety and
effectiveness for the treatment of anatomic regions other than the
mid-face with JUVÉDERM® VOLUMA™ XC; facial
wrinkles and folds with JUVÉDERM® Ultra XC,
JUVÉDERM® Ultra Plus
XC, and JUVÉDERM® VOLLURE™
XC; and the lips and perioral area with JUVÉDERM® Ultra XC
and JUVÉDERM® VOLBELLA™ XC have
not been established in controlled clinical studies
- As with all
transcutaneous procedures, dermal filler implantation carries a risk of
infection. Follow standard precautions associated with injectable
materials
- The safety for
use during pregnancy, in breastfeeding females, and in patients with known
susceptibility to keloid formation, hypertrophic scarring, and pigmentation
disorders has not been studied
- The safety for
use of JUVÉDERM® VOLUMA™ XC in
patients under 35 or over 65 years, JUVÉDERM® Ultra XC
and JUVÉDERM® Ultra Plus
XC in patients under 18 years, and JUVÉDERM® VOLLURE™
XC and JUVÉDERM® VOLBELLA™ XC in patients
under 22 years has not been established
- Use with caution
in patients on immunosuppressive therapy
- Patients who are
using products that can prolong bleeding (such as aspirin, nonsteroidal
anti-inflammatory drugs, and warfarin) may experience increased bruising
or bleeding at treatment sites
- If laser
treatment, chemical peel, or any other procedure based on active dermal
response is considered after treatment, or if these products are
administered before the skin has healed completely, there is a possible
risk of an inflammatory reaction at the treatment site
- Patients who
experience skin injury near the site of implantation may be at a higher
risk for adverse events
- The safety of
JUVÉDERM® VOLUMA™ XC
injectable gel for use in patients with very thin skin in the mid-face and
Fitzpatrick Skin Types V and VI has not been established
- Patients may
experience late onset nodules with use of dermal fillers including
JUVÉDERM® VOLUMA™ XC
- Patients may
experience late onset adverse events with use of dermal fillers
ADVERSE EVENTS
The most commonly
reported side effects for JUVÉDERM® injectable
gels were injection-site redness, swelling, pain, tenderness, firmness,
lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA™ XC,
dryness was also reported. For JUVÉDERM® VOLUMA™ XC,
side effects were predominantly moderate in severity, with duration of 2 to 4
weeks; for JUVÉDERM® Ultra XC , JUVÉDERM® Ultra
Plus XC, or JUVÉDERM® VOLLURE™ XC, they
were mostly mild or moderate in severity, with duration of 14 days or less; and
for JUVÉDERM® VOLBELLA™ XC,
they were predominantly mild or moderate, with duration of 30 days or less.
To report an adverse reaction with any product in the JUVÉDERM® Collection, please call Allergan at 1-800-433-8871. Please visit JuvedermDFU.com for more information.
Products in the JUVÉDERM® Collection are available by prescription only.
Nikki Carmen - Facebook Review
Johns Creek Derm... did my filler and I’m absolutely in love! Highly recommend them for any cosmetic needs!!
PATIENT REVIEWS