Peggy Kathleen - Google Review
I have been a client of the lovely Dr. Timani for a few years, and have seen her at both offices for cosmetic and medical needs. Dr. Timani’s talent, education and attention to detail have created a very loyal client. She is an artist! If anything is ever not quite right she makes sure to adjust as needed. You get what you pay for!
PATIENT REVIEWS


Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan KYBELLA®
KYBELLA® (deoxycholic
acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic
acid) injection is indicated for improvement in the appearance of moderate to severe
convexity or fullness associated with submental fat in adults.
The
safe and effective use of KYBELLA® for the treatment of
subcutaneous fat outside the submental region has not been established and is
not recommended.
IMPORTANT
SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is
contraindicated in the presence of infection at the injection sites.
WARNINGS
AND PRECAUTIONS
Marginal
Mandibular Nerve Injury
Cases
of marginal mandibular nerve injury, manifested as an asymmetric smile or
facial muscle weakness, were reported in 4% of subjects in the clinical trials;
all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA®
should not be injected into or in close proximity to the marginal mandibular
branch of the facial nerve.
Dysphagia
Dysphagia
occurred in 2% of subjects in the clinical trials in the setting of
administration-site reactions, eg, pain, swelling, and induration of the
submental area; all cases of dysphagia resolved spontaneously (range 1-81 days,
median 3 days). Avoid use of KYBELLA® in patients with current
or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site
Hematoma/Bruising
In
clinical trials, 72% of subjects treated with KYBELLA® experienced
hematoma/bruising. KYBELLA® should be used with caution in
patients with bleeding abnormalities or who are currently being treated with
antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the
treatment area may occur.
Risk
of Injecting into or in Proximity to Vulnerable Anatomic Structures
To
avoid the potential of tissue damage, KYBELLA® should not be
injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph
nodes, and muscles. Care should be taken to avoid inadvertent injection
directly into an artery or a vein as it can result in vascular injury
Injection
Site Alopecia
Cases
of injection site alopecia have been reported with administration of KYBELLA®.
Onset and duration may vary among individuals and may persist. Consider
withholding subsequent treatments until resolution.
Injection
Site Ulceration and Necrosis
Injections
that are too superficial into the dermis may result in skin ulceration and
necrosis. Cases of injection site ulceration and necrosis have been reported
with administration of KYBELLA®. Do not administer KYBELLA® into
affected area until complete resolution.
ADVERSE
REACTIONS
The
most commonly reported adverse reactions in the pivotal clinical trials were:
injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and
induration.
Please
see KYBELLA® full Prescribing Information.
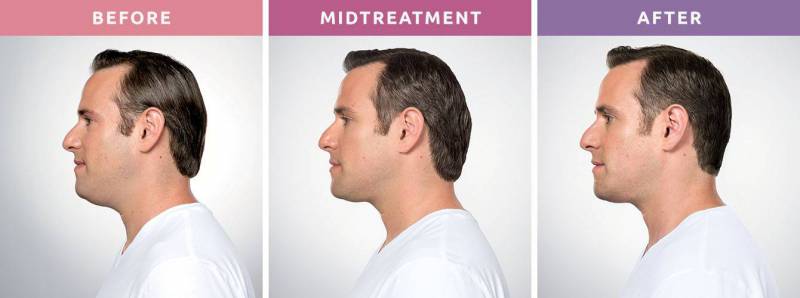
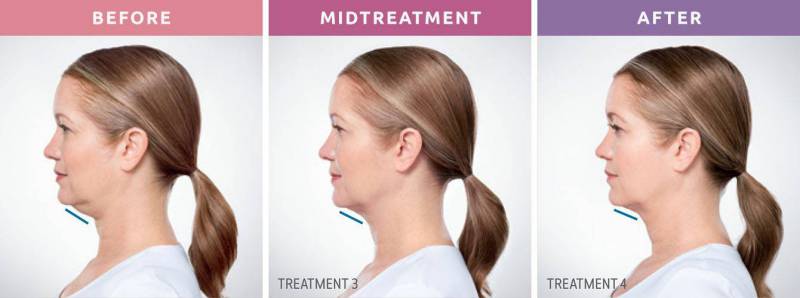
Say Goodbye to Submental Fullness—Without Surgery
Notice a little extra volume beneath your chin? Whether it’s something you’ve always had, developed over time, or recently started to see in photos, this under-the-chin fullness—often called a double chin—can affect men and women alike. This condition, known medically as submental fullness, may be caused by:
• Genetics
• Aging
• Weight changes
Even if you're at a healthy weight and follow a balanced lifestyle, submental fullness can persist. That's where KYBELLA may help.
What Is KYBELLA®?
KYBELLA is an FDA-approved injectable treatment that targets and permanently destroys fat cells under the chin. It's the first and only non-surgical injectable designed specifically to reduce moderate to severe submental fullness. Unlike other treatments that require incisions or downtime, KYBELLA uses deoxycholic acid, a molecule naturally found in the body, to break down and absorb fat in the treated area. Once the fat cells are destroyed, they cannot return.
How KYBELLA® Works
• Quick in-office injections under the chin
• Minimal discomfort, with treatments taking only about 15–20 minutes
• Gradual, natural-looking improvement in your profile
• Most patients see results after 2–4 sessions (some may need up to 6)
As the fat is metabolized, your chin profile becomes more defined, balanced, and youthful, without the need for surgery or downtime.
Why Choose Johns Creek Dermatology for KYBELLA®
At Johns Creek Dermatology, we bring advanced dermatologic expertise and personalized care to every KYBELLA treatment:
Board-Certified Experts
Our experienced dermatology team ensures that your treatment is safe, precise, and tailored to your facial structure.
Customized Treatment Plans
No two chins are the same. We design your KYBELLA journey around your aesthetic goals and facial anatomy.
Combinable with Other Treatments
Looking to enhance results? We may recommend pairing KYBELLA with NovaThreads for additional lift and contouring—without surgery.
Transparent, Patient-Centered Care
We walk you through every step—from consultation to aftercare—so you feel informed, empowered, and confident in your decision.
Proven, Lasting Results
KYBELLA offers permanent fat reduction in the treated area, backed by science and FDA approval.
What to Expect After KYBELLA®
It’s normal to experience mild swelling, numbness, or bruising under the chin following treatment. These effects usually subside within 2 to 3 days. Cold compresses can help reduce discomfort.
Visible Results in 2–4 Sessions
While individual results vary, many patients begin to notice improvements after just 2–4 treatments. In clinical trials, 59% of patients received up to 6 sessions for optimal results.
Is KYBELLA® Right for You?
You may be a good candidate for KYBELLA if:
• You're bothered by fullness under the chin
• You feel your profile makes you look older or heavier
• You want a non-surgical option for chin contouring
• You maintain a healthy lifestyle, but the fullness won’t go away
Redefine Your Profile On Your Terms
Ready to explore a non-surgical way to contour your chin and restore confidence in your profile? Schedule a consultation with our expert dermatology team at Johns Creek Dermatology today.
Serving Johns Creek, Alpharetta, Duluth, Suwanee, Roswell, and the North Atlanta area
Frequently Asked Questions
How long do the results last?
The results are permanent. Once dissolved, these cells can no longer store or accumulate fat, so further treatment is not expected once you reach your desired aesthetic goal.
How many treatments will I need?
Since everyone’s chin profile is different, the number of treatments varies from patient to patient.
Does it hurt?
Each patient will have their own pain threshold. Patients have reported that KYBELLA® injections are no more than a mild burning sensation for around 15 minutes. We apply ice packs for this time to maximize our patients' comfort.
What is the downtime?
After KYBELLA® treatment, you will likely have some swelling, bruising, or numbness under your chin that will subside within 2 to 3 days. Cold compresses may be used to reduce the swelling.
Is KYBELLA® safe?
KYBELLA® is a prescription medicine and is the only FDA-approved injectable treatment used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin.”
Visit Kyeblla website more information.
Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan KYBELLA®
KYBELLA® (deoxycholic
acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic
acid) injection is indicated for improvement in the appearance of moderate to severe
convexity or fullness associated with submental fat in adults.
The
safe and effective use of KYBELLA® for the treatment of
subcutaneous fat outside the submental region has not been established and is
not recommended.
IMPORTANT
SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is
contraindicated in the presence of infection at the injection sites.
WARNINGS
AND PRECAUTIONS
Marginal
Mandibular Nerve Injury
Cases
of marginal mandibular nerve injury, manifested as an asymmetric smile or
facial muscle weakness, were reported in 4% of subjects in the clinical trials;
all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA®
should not be injected into or in close proximity to the marginal mandibular
branch of the facial nerve.
Dysphagia
Dysphagia
occurred in 2% of subjects in the clinical trials in the setting of
administration-site reactions, eg, pain, swelling, and induration of the
submental area; all cases of dysphagia resolved spontaneously (range 1-81 days,
median 3 days). Avoid use of KYBELLA® in patients with current
or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site
Hematoma/Bruising
In
clinical trials, 72% of subjects treated with KYBELLA® experienced
hematoma/bruising. KYBELLA® should be used with caution in
patients with bleeding abnormalities or who are currently being treated with
antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the
treatment area may occur.
Risk
of Injecting into or in Proximity to Vulnerable Anatomic Structures
To
avoid the potential of tissue damage, KYBELLA® should not be
injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph
nodes, and muscles. Care should be taken to avoid inadvertent injection
directly into an artery or a vein as it can result in vascular injury
Injection
Site Alopecia
Cases
of injection site alopecia have been reported with administration of KYBELLA®.
Onset and duration may vary among individuals and may persist. Consider
withholding subsequent treatments until resolution.
Injection
Site Ulceration and Necrosis
Injections
that are too superficial into the dermis may result in skin ulceration and
necrosis. Cases of injection site ulceration and necrosis have been reported
with administration of KYBELLA®. Do not administer KYBELLA® into
affected area until complete resolution.
ADVERSE
REACTIONS
The
most commonly reported adverse reactions in the pivotal clinical trials were:
injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and
induration.
Please
see KYBELLA® full Prescribing Information.
Peggy Kathleen - Google Review
I have been a client of the lovely Dr. Timani for a few years, and have seen her at both offices for cosmetic and medical needs. Dr. Timani’s talent, education and attention to detail have created a very loyal client. She is an artist! If anything is ever not quite right she makes sure to adjust as needed. You get what you pay for!
PATIENT REVIEWS