CALL US TO BOOK AN APPOINTMENT
(770) 771-6591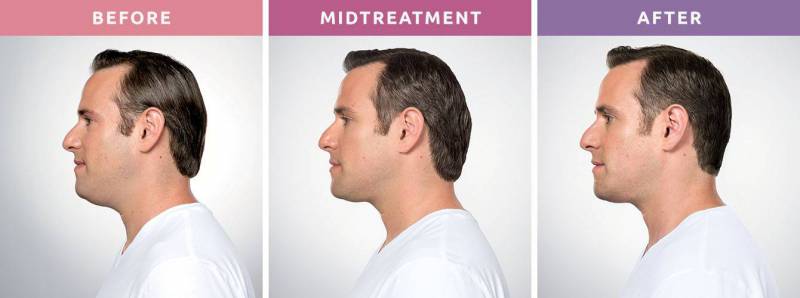


Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan KYBELLA®
KYBELLA® (deoxycholic
acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic
acid) injection is indicated for improvement in the appearance of moderate to severe
convexity or fullness associated with submental fat in adults.
The
safe and effective use of KYBELLA® for the treatment of
subcutaneous fat outside the submental region has not been established and is
not recommended.
IMPORTANT
SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is
contraindicated in the presence of infection at the injection sites.
WARNINGS
AND PRECAUTIONS
Marginal
Mandibular Nerve Injury
Cases
of marginal mandibular nerve injury, manifested as an asymmetric smile or
facial muscle weakness, were reported in 4% of subjects in the clinical trials;
all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA®
should not be injected into or in close proximity to the marginal mandibular
branch of the facial nerve.
Dysphagia
Dysphagia
occurred in 2% of subjects in the clinical trials in the setting of
administration-site reactions, eg, pain, swelling, and induration of the
submental area; all cases of dysphagia resolved spontaneously (range 1-81 days,
median 3 days). Avoid use of KYBELLA® in patients with current
or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site
Hematoma/Bruising
In
clinical trials, 72% of subjects treated with KYBELLA® experienced
hematoma/bruising. KYBELLA® should be used with caution in
patients with bleeding abnormalities or who are currently being treated with
antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the
treatment area may occur.
Risk
of Injecting into or in Proximity to Vulnerable Anatomic Structures
To
avoid the potential of tissue damage, KYBELLA® should not be
injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph
nodes, and muscles. Care should be taken to avoid inadvertent injection
directly into an artery or a vein as it can result in vascular injury
Injection
Site Alopecia
Cases
of injection site alopecia have been reported with administration of KYBELLA®.
Onset and duration may vary among individuals and may persist. Consider
withholding subsequent treatments until resolution.
Injection
Site Ulceration and Necrosis
Injections
that are too superficial into the dermis may result in skin ulceration and
necrosis. Cases of injection site ulceration and necrosis have been reported
with administration of KYBELLA®. Do not administer KYBELLA® into
affected area until complete resolution.
ADVERSE
REACTIONS
The
most commonly reported adverse reactions in the pivotal clinical trials were:
injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and
induration.
Please
see KYBELLA® full Prescribing Information.
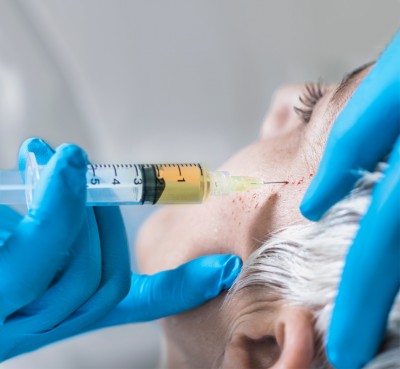
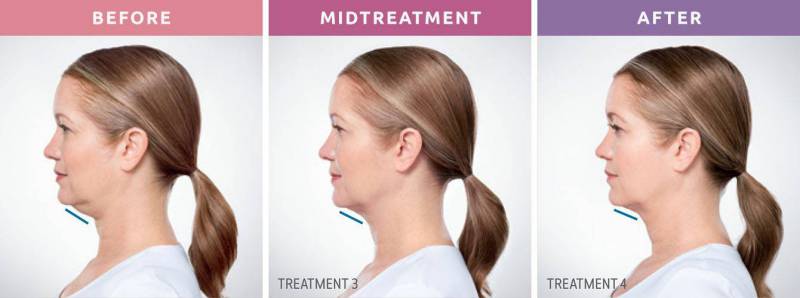
A neckline you can be proud of! No more double chin.
The “first and only” groundbreaking injectable treatment, Kybella is a nonsurgical option to permanently remove fat cells and pockets below the chin. FDA approved, it gets rid of the excess fat in the submental area that creates a double chin and affects the slope of your profile. Because a double chin is caused by genetics, aging, and weight gain, diet and exercise may not help. But Kybella at Johns Creek Dermatology in Johns Creek North Atlanta can!
What makes Kybella an effective double chin removal
treatment is the active ingredient, Deoxycholic acid, a naturally occurring
molecule that actively breaks down fat in the body. Multiple treatments may be
required, but the treatments are quick with minimal discomfort.
This treatment can be combined with NovaThreads.
Our dermatologist at Johns Creek Dermatology is ready to assess your needs for optimal results and to discuss all the risks and benefits.
For more information visit
https://www.mykybella.com/patient-stories
What is submental fullness?
Submental fullness is a gathering of excess fat beneath the chin, commonly known as “double chin.” It can be something you’ve had your whole life due to genetics or start to appear as you get older or if you gain weight.
What is KYBELLA®?
The active ingredient in KYBELLA® is synthetic deoxycholic acid. Deoxycholic acid is a naturally occurring molecule in the body that aids in the breakdown and absorption of dietary fat. When injected into the fat beneath the chin, KYBELLA® destroys fat cells and once destroyed, these cells can no longer store or accumulate fat. This results in a noticeable reduction in fullness under the chin, revealing an improved chin profile.
When will I see the results?
Visible results in 2-4 treatments. The results of KYBELLA® injections happen gradually. You will start noticing changes in your appearance within 4 to 6 weeks. However, it typically takes about 3 months before you can see the full results.
How long do the results last?
The results are permanent. Once dissolved, these cells can no longer store or accumulate fat, so further treatment is not expected once you reach your desired aesthetic goal.
How many treatments will I need?
Since everyone’s chin profile is different, the number of treatments varies from patient to patient.
Does it hurt?
Each patient will have their own pain threshold. Patients have reported that KYBELLA® injections are no more than a mild burning sensation for around 15 minutes. We apply ice packs for this time to maximize our patients' comfort.
What is the downtime?
After KYBELLA® treatment, you will likely have some swelling, bruising, or numbness under your chin that will subside within 2 to 3 days. Cold compresses may be used to reduce the swelling.
Is KYBELLA® safe?
KYBELLA® is a prescription medicine and is the only FDA-approved injectable treatment used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin.”




Indications, Important Safety Information and Prescribing Information
All images © 2020 Allergan KYBELLA®
KYBELLA® (deoxycholic
acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic
acid) injection is indicated for improvement in the appearance of moderate to severe
convexity or fullness associated with submental fat in adults.
The
safe and effective use of KYBELLA® for the treatment of
subcutaneous fat outside the submental region has not been established and is
not recommended.
IMPORTANT
SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is
contraindicated in the presence of infection at the injection sites.
WARNINGS
AND PRECAUTIONS
Marginal
Mandibular Nerve Injury
Cases
of marginal mandibular nerve injury, manifested as an asymmetric smile or
facial muscle weakness, were reported in 4% of subjects in the clinical trials;
all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA®
should not be injected into or in close proximity to the marginal mandibular
branch of the facial nerve.
Dysphagia
Dysphagia
occurred in 2% of subjects in the clinical trials in the setting of
administration-site reactions, eg, pain, swelling, and induration of the
submental area; all cases of dysphagia resolved spontaneously (range 1-81 days,
median 3 days). Avoid use of KYBELLA® in patients with current
or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site
Hematoma/Bruising
In
clinical trials, 72% of subjects treated with KYBELLA® experienced
hematoma/bruising. KYBELLA® should be used with caution in
patients with bleeding abnormalities or who are currently being treated with
antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the
treatment area may occur.
Risk
of Injecting into or in Proximity to Vulnerable Anatomic Structures
To
avoid the potential of tissue damage, KYBELLA® should not be
injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph
nodes, and muscles. Care should be taken to avoid inadvertent injection
directly into an artery or a vein as it can result in vascular injury
Injection
Site Alopecia
Cases
of injection site alopecia have been reported with administration of KYBELLA®.
Onset and duration may vary among individuals and may persist. Consider
withholding subsequent treatments until resolution.
Injection
Site Ulceration and Necrosis
Injections
that are too superficial into the dermis may result in skin ulceration and
necrosis. Cases of injection site ulceration and necrosis have been reported
with administration of KYBELLA®. Do not administer KYBELLA® into
affected area until complete resolution.
ADVERSE
REACTIONS
The
most commonly reported adverse reactions in the pivotal clinical trials were:
injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and
induration.
Please
see KYBELLA® full Prescribing Information.